Jared Augenstein’s articles from Manatt, Phelps & Phillips LLP are most popular:
- within Food, Drugs, Healthcare and Life Sciences topic(s)
- with readers working within the Automotive and Property industries
Manatt, Phelps & Phillips LLP are most popular:
- within Real Estate and Construction, Immigration, Litigation and Mediation & Arbitration topic(s)
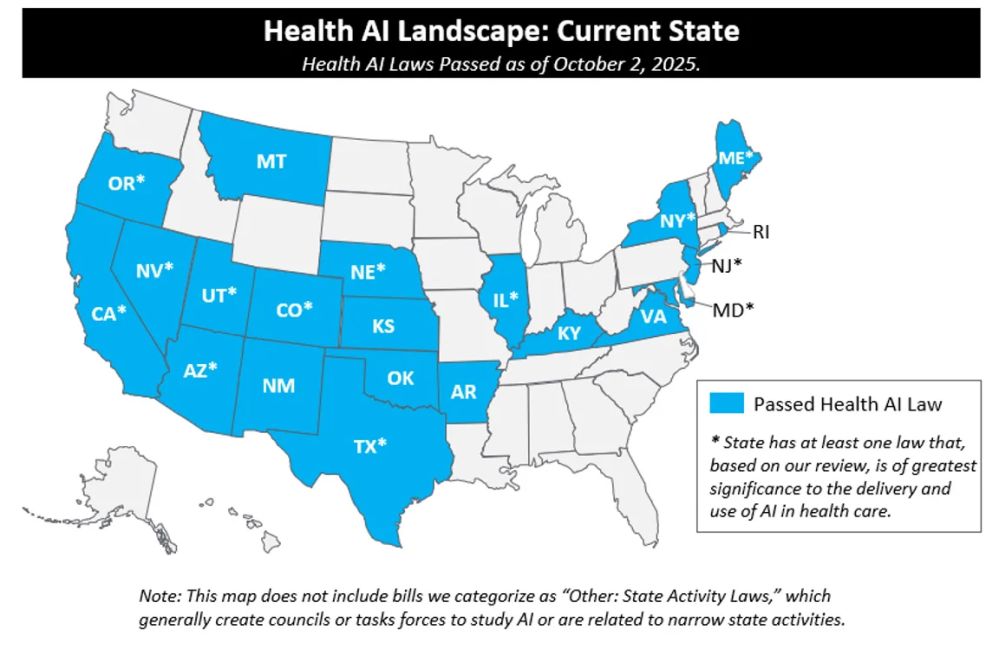
Activity on AI in health care has been at the forefront of the AI debate during the 2025 state legislative sessions, and is increasingly being discussed at the federal level. As of the end of Q3:
- 47 states introduced AI bills impacting health care
- 21 states signed 33 AI bills impacting health care into law
- Over 250 AI bills impacting health care were introduced across the country
Actors across all levels – state, federal, and self-regulating bodies and accreditation organizations – are defining and issuing guidance governing the development and use of AI in health care:
- State Action: Despite most legislative sessions ending by early in Q3, there was substantive action across four AI focus areas: use of AI enabled chatbots, AI in clinical care, AI use by payors, and transparency, safety, and disclosure governance requirements for AI developers and deployers. California and Illinois passed significant legislation this quarter.
- Federal Activity: In the latter half of Q3, the White House and Congress increased their role in conversation regarding AI in health care, including an Executive Order and Request for Information from the White House; Senator Cruz' (R – Texas) introduction of the SANDBOX Act to create a pathway for waivers from Federal regulation governing AI innovation; and a congressional hearing exploring AI's potential, including to streamline clinical trials and reduce administrative burden in health care. Federal agencies were similarly active, including the FTC, FDA, and CMS, advancing AI-focused inquiries and oversight activities; issuing agency-specific guidance on the use of AI; and convening expert groups on implementation of AI.
- Self-Regulating Bodies and Accreditation Organizations: Q3 2025 saw increased guidance issued and action from self-regulating bodies and accreditation organizations supplement the patchwork of existing state and federal regulation, including AI-focused accreditation tracks from the Utilization Review Accreditation Commission (URAC), guidance from Joint Commission and the Coalition for Health AI (CHAI), and an AI workgroup convened by the National Committee for Quality Assurance (NCQA).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.






